- Dan Falk from In Search of Time: The History, Physics, and Philosophy of Time
| In the book, Burkeman makes the point that we often want to rationalize that the hard work we do today will pay off in the future. And there is some truth to this. But, he also mentions we wrongly believe that through our present efforts and sacrifices - the checking off of our "to-do list" - we will ultimately reach some idealized future where we will magically "have more time" and then be able to focus on what really matters. |
Yesterday is history. Tomorrow is a mystery. Today is a gift, that is why they call it the Present.
May by a bit trite but that doesn't mean there is not wisdom in it.
It speaks to the idea that the present is within our control as it is happening now.
| And while a lot of this memory dividend talk brings to mind spending lavishly on a multi-thousand dollar African Safari, Mediterranean Cruise, or week-long trip to Disney World, one doesn't have to spend a lot of money to make memories with family and friends. In a way, being present with those we care about can be enough. Making the time for them and prioritizing that over other commitments can be everything. Ironically, we often remember some of the smallest, seemingly mundane experiences with those we love as strongly as the big, audacious trips and outings. And these memories often become more powerful with the passing of time and, ultimately, sadly, when those we made them with are no longer with us. Then, the memory is what is left in the end. A reminder of that individual and their impact on you and you on them. |
- The power of human connection
- Why you should get involved in things outside the lab/work
- Cultivate serendipity by giving back and getting involved
- Find your passion? Finding meaning and purpose in your work and life
For Further Reading:
- The Science of Mental Time Travel and Why Our Ability to Imagine the Future Is Essential to Our Humanity
- In Search of Time: The History, Physics, and Philosophy of Time (Book)
- Your Brain is a Time Machine (Book)
- Die With Zero (Book)
Watch:












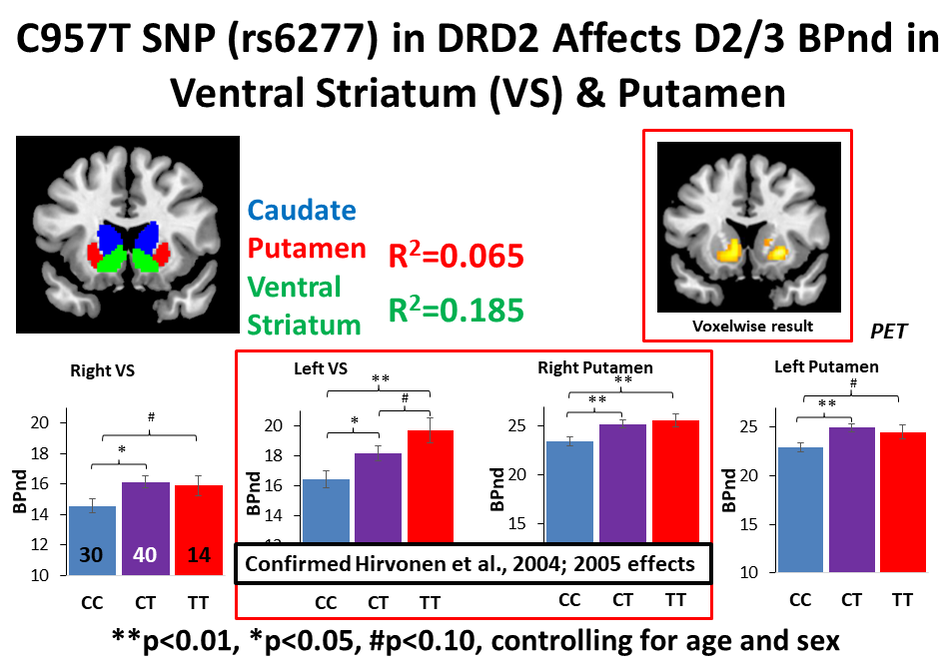
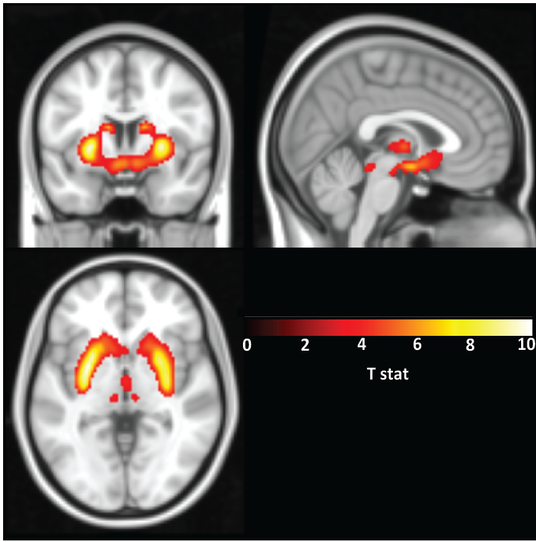

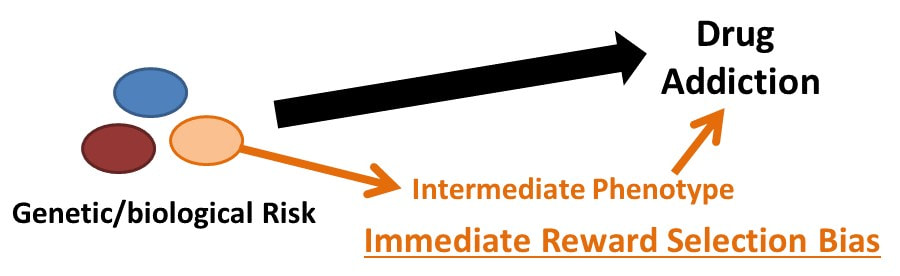
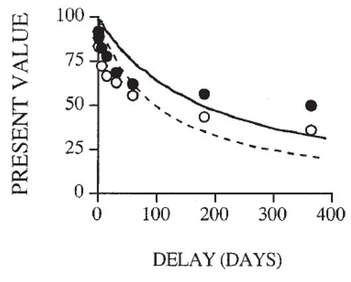

 RSS Feed
RSS Feed