Dopamine signaling plays a role in a variety of critical cognitive processes including motor control, learning, and decision making. It has also been implicated in the addictive nature of drugs of abuse, which I studied in some detail during my Ph.D. and postdoctoral research.
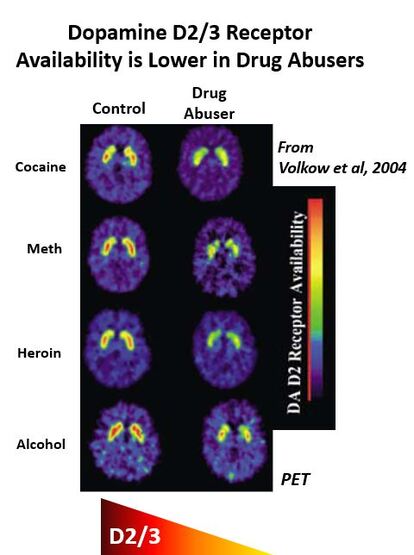
PET imaging has shown that lower dopamine D2/3 receptors are present in a variety of drug-addicted individuals (alcohol, cocaine, methamphetamine, heroin) when compared to healthy controls. Whether low D2 receptors are a cause or consequence of problematic drug use has been difficult to determine in human studies, however.
- Taq1A - A1 allele associated with lower striatal D2 receptor availability (replicated in separate study but not in a third)
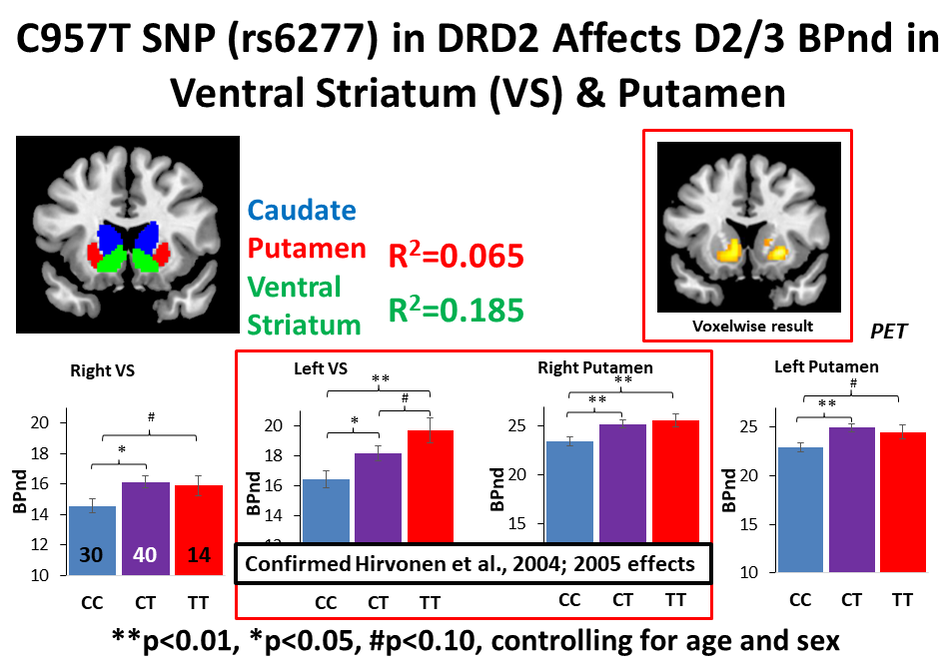
- C957T - C allele associated with lower striatal D2 receptor availability in study of 45 individuals
- -141C Ins/Del - inconsistent findings on whether it affects D2 receptor availability
The C957T and -141C Ins/Del polymorphisms are in strong linkage disequilibrium with Taq1A and have themselves been associated with striatal D2/3 receptor availability. Despite the data suggesting that these SNPs are strongly linked, few studies have systematically investigated the effect of C957T, -141C Ins/Del, and Taq1A in isolation and combination on D2/3 receptor availability. Beyond the potential link to drug addiction risk, characterizing the functional effect of these SNPs on D2/3 receptor availability has implications for better understanding the mechanisms through which they exert their demonstrated influence on motivated behaviors including learning and decision making, impulsivity, and reward responsivity.
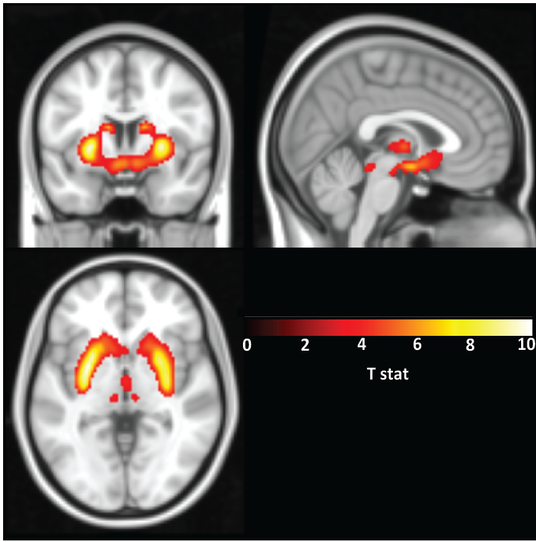
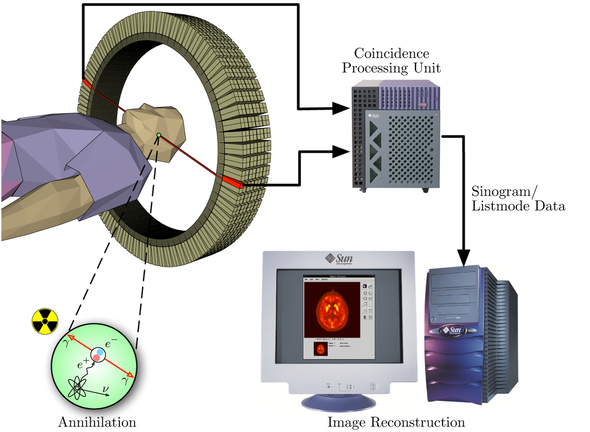
In our work, we used F-18-Fallypride, which is a D2/3 receptor tracer with favorable affinity to measure both striatal and extrastriatal dopamine receptors, and assessed the impact of C957T, Taq1A and -141C Ins/Del SNPs on D2/3 receptor availability in a sample of 84 healthy subjects.
Read more about wanting, liking, and drug abuse in a previous blog post.
The concept of blunted dopamine signaling (lower D2 receptor levels and less dopamine release) as biomarkers of addiction has also been recently reviewed (Trifilieff et al 2017; Leyton, 2017). While more work needs to be done, understanding factors that influence these PET-based biomarkers of dopamine signaling in human subjects has the potential to identify at risk individuals. This risk identification may allow intervention to be attempted earlier in the addiction process or perhaps prevent addiction before it even occurs.
My own work, referenced above, suggests that genetic variation in a gene encoding the dopamine D2 receptor (DRD2) can affect the relative availability of this receptor in the brain as measured with PET (Smith et al., 2017 Translational Psychiatry). Individuals with a particular genetic variant in DRD2 that is associated with less availability of the receptor (C957T CC individuals) may need either a higher dose of a D2 drug or a higher affinity D2 drug to receive a therapeutic benefit.
The implications for this finding go beyond potential treatments or interventions for drug addiction. D2 agonists are commonly used in Parkinson’s Disease patients to preserve motor function and D2 antagonist-like drugs are used in the treatment of Schizophrenia. Understanding the genotype of individuals affected with these conditions, then, could enhance the effectiveness of their D2 drug treatments (by suggesting a physician might want to start with a higher or lower dose of the drug). While studies such as ours linking genetic variation with differences in biology are encouraging, DNA can also be modified by the environment. Researchers have begun studying these epigenetic effects on behavior, with most work occurring in rodents. As we integrate this knowledge, we will begin to better understand the impact gene by environment interactions have on biology and behavior.
Our quest to better understand individual differences, however, has the potential to lead to more targeted treatments and therapies for a variety of dopamine-associated disorders including ADHD, Schizophrenia, Parkinson’s Disease, and drug addiction. The development of these personalized treatments will undoubtedly improve healthcare in the 21st Century and beyond but will require further research focused on measuring and categorizing individual differences.
- Declining Dopamine: How aging affects a key modulator of reward processing and decision making
- Stress & the Brain: How genetics affects whether you are more likely to wilt under pressure
- Wanting, Liking, & Dopamine's Role in Addiction
- Now vs Later - How immediate reward selection bias may be a risk factor for addiction
More scholarly articles on dopamine and its effects:

 RSS Feed
RSS Feed